Reactivity of NHC/diphosphene-coordinated Au(i)-hydride†
Abstract
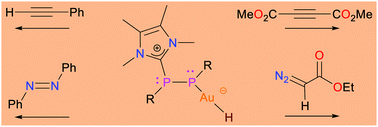
We report the reactivity of isolable Au(I)-hydride stabilized by an NHC-coordinated diphosphene towards substrates containing C–C and N–N multiple bonds (NHC = N-heterocyclcic carbene). Reactions with dimethyl acetylenedicarboxylate and azobenzene lead to a trans-addition of the Au(I)-H across the C–C triple bond and the N–N double bond, respectively. In contrast, the reaction with ethyl diazoacetate affords a gold(I)-hydrazonide as the 1,1-addition product to the terminal nitrogen atom. With phenyl acetylene, the corresponding Au(I)-alkynyl complex is obtained under the elimination of dihydrogen. Strikingly, diphosphene-containing Au(I)-hydride is more reactive – affording different products in some cases – than a related NHC-stabilized Au(I)-hydride without the mediating diphosphene moiety.


 Please wait while we load your content...
Please wait while we load your content...