Efficient construction of diverse 3-cyanoindoles under novel tandem catalysis†
Abstract
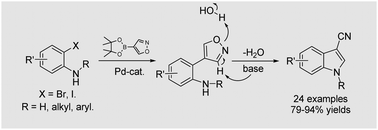
A novel and rapid construction of 3-cyanoindoles by palladium-catalyzed tandem reactions has been developed. “N–H” free unprotected, N-alkyl and N-aryl 3-cyanoindoles are obtained with good to excellent yields. The usefulness of this synthetic approach is further demonstrated by the successful synthesis of practical compounds such as the therapeutic estrogen receptor ligand A precursor. Mechanism study shows that the tandem catalysis exploits a Suzuki cross-coupling with subsequent base-induced isoxazole fragmentation, followed by the aldimine condensation.


 Please wait while we load your content...
Please wait while we load your content...