Designing a Cr-catalyst bearing redox non-innocent phenalenyl-based ligand towards hydrosilylative CO2 functionalization†‡
Abstract
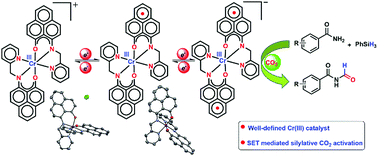
Herein, we report the synthesis of a Cr(III)-complex bearing a redox non-innocent phenalenyl-based ligand and its use as a catalyst for SET mediated hydrosilylative reduction of carbon dioxide towards formylation of primary amides under mild conditions. A preliminary mechanistic picture for this transformation has been proposed by isolation and characterization of several reactive intermediates.


 Please wait while we load your content...
Please wait while we load your content...