Photoinduced umpolung addition of carbonyl compounds with α,β-unsaturated esters enables the polysubstituted γ-lactone formation†
Abstract
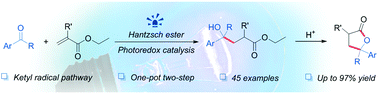
We herein report the photoinduced intermolecular umpolung addition of aromatic ketones/aldehydes with α,β-unsaturated esters via ketyl radical intermediates. Following an intramolecular transesterification, a variety of γ-lactone derivatives are readily accessed. Mechanistic investigations demonstrate the significant role of Hantzsch ester, which serves both as the electron and proton donor.


 Please wait while we load your content...
Please wait while we load your content...