H2 evolution from H2O via O–H oxidative addition across a 9,10-diboraanthracene†
Abstract
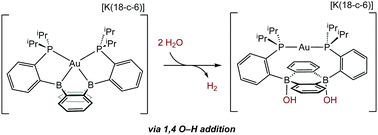
The water reactivity of the boroauride complex ([Au(B2P2)][K(18-c-6)]; (B2P2, 9,10-bis(2-(diisopropylphosphino)-phenyl)-9,10-dihydroboranthrene) and its corresponding two-electron oxidized complex, Au(B2P2)Cl, are presented. Au(B2P2)Cl is tolerant to H2O and forms the hydroxide complex Au(B2P2)OH in the presence of H2O and triethylamine. [Au(B2P2)]Cl and [Au(B2P2)]OH are poor Lewis acids as judged by the Gutmann–Becket method, with [Au(B2P2)]OH displaying facile hydroxide exchange between B atoms of the DBA ring as evidenced by variable temperature NMR spectroscopy. The reduced boroauride complex [Au(B2P2)]− reacts with 1 equivalent of H2O to produce a hydride/hydroxide product, [Au(B2P2)(H)(OH)]−, that rapidly evolves H2 upon further H2O reaction to yield the dihydroxide compound, [Au(B2P2)(OH)2]−. [Au(B2P2)]Cl can be regenerated from [Au(B2P2)(OH)2]−via HCl·Et2O, providing a synthetic cycle for H2 evolution from H2O enabled by O–H oxidative addition at a diboraanthracene unit.


 Please wait while we load your content...
Please wait while we load your content...