Rotational dynamics of the imidazolium ion in cyanide-bridged dielectric framework materials†
Abstract
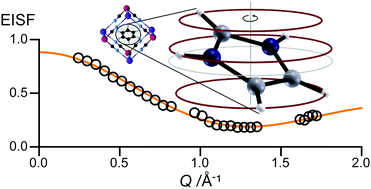
Reorientation of organic cations in the cubic interstices of cyanoelpasolite molecular perovskites results in a variety of structural phase transitions, but far less is known about these cations’ dynamics. We report quasielastic neutron scattering from the materials (C3H5N2)2K[MIII(CN)6], M = Fe,Co, which is directly sensitive to the rotation of the imidazolium ion. The motion is well described by a circular three-site hopping model, with the ion rotating within its plane in the intermediate-temperature phase, but tilting permanently in the high-temperature phase. Thus the two rhombohedral phases, which are crystallographically rather similar, have markedly different dynamics. The activation energy of rotation is about 10 kJ mol−1 and the barrier between orientations is 6 kJ mol−1. Our results explain two anomalous features in these materials’ dielectric constants.
- This article is part of the themed collection: Functional Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...