Using the FpXylBH2•SMe2 reagent for the regioselective synthesis of cyclic bis(alkenyl)boranes†
Abstract
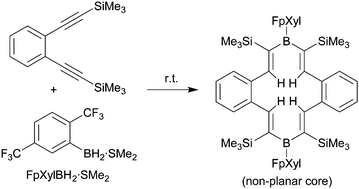
The reactive borane reagent FpXylBH2•SMe2 was prepared from 1,4-bis(trifluoromethyl)benzene by treatment with n-BuLi, followed by H3B·SMe2 and subsequent removal of hydride. It undergoes a regioselective hydroboration reaction with 1,2-bis(trimethylsilylethynyl)benzene to give the “dimeric” product 13a featuring a conjugated 14-membered core heterocyclic structure that contains a pair of FpXylB units.


 Please wait while we load your content...
Please wait while we load your content...