Exploiting hexafluoroisopropanol (HFIP) in Lewis and Brønsted acid-catalyzed reactions
Abstract
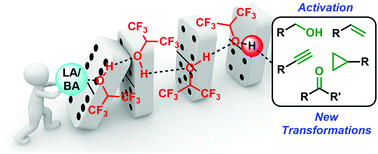
Hexafluoroisopropanol (HFIP) is a solvent with unique properties that has recently gained attention for promoting a wide range of challenging chemical reactions. It was initially believed that HFIP was almost exclusively involved in the stabilization of cationic intermediates, owing to its high polarity and low nucleophilicity. However, in many cases, the mechanism of action of HFIP appears to be more complex. Recent findings reveal that many Lewis and Brønsted acid-catalyzed transformations conducted in HFIP additionally involve cooperation between the catalyst and HFIP hydrogen-bond clusters, akin to Lewis- or Brønsted acid-assisted-Brønsted acid catalysis. This feature article showcases the remarkable versatility of HFIP in Lewis and Brønsted acid-catalyzed reactions, with an emphasis on examples yielding mechanistic insight.


 Please wait while we load your content...
Please wait while we load your content...