Biomimetic systems involving sequential redox reactions in glycolysis – the sulfur effect†
Abstract
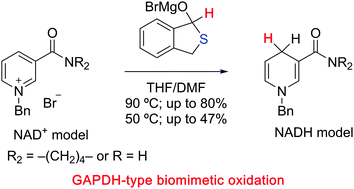
Magnesium hemithioacetates were used as model cysteine compounds to mimic natural hemithioacetals, and their biomimetic oxidation reactions using a model NAD+ compound were investigated. Cyclic hemithioacetate was found to be the best substrate for the reaction with the model NAD+ compound, which gave the corresponding NADH analog in excellent yield.


 Please wait while we load your content...
Please wait while we load your content...