Mobility of Lewis acids within the secondary coordination sphere: toward a model for cooperative substrate binding†
Abstract
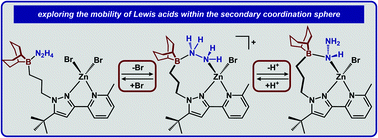
Distance dependence of appended Lewis acids in N2H4 binding and deprotonation was evaluated within a series of zinc complexes. Variation of spacer-length to a tethered trialkylborane Lewis acid revealed distinct preferences for binding and stabilization of the resulting deprotonated N2H3− unit.
- This article is part of the themed collection: Bioinspired metal complexes for chemical transformations and catalysis


 Please wait while we load your content...
Please wait while we load your content...