Synthesis of polycyclic indoles via organocatalytic bicyclization of α-alkynylnaphthalen-2-ols with nitrones†
Abstract
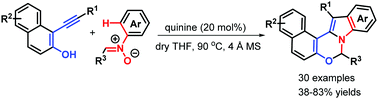
A new organocatalytic bicyclization of α-alkynylnaphthalen-2-ols with nitrones was first reported, leading to the convergent synthesis of polycyclic indoles with substantial substitution diversity in generally good yields through scission of the N–O bond of nitrones via [3,3]-sigmatropic rearrangement. This transformation showcases the use of a quinine catalyst in a complicated cascade system that has been shown to effectively construct polycyclic heterocycles via alkyne difunctionalization.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...