Iron-catalysed enantioconvergent Suzuki–Miyaura cross-coupling to afford enantioenriched 1,1-diarylalkanes†
Abstract
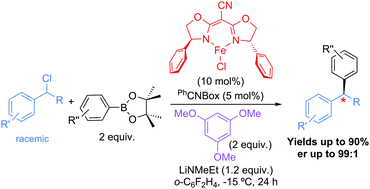
The first stereoconvergent Suzuki–Miyaura cross-coupling reaction was developed to afford enantioenriched 1,1-diarylalkanes. An iron-based complex containing a chiral cyanobis(oxazoline) ligand framework was best to obtain enantioenriched 1,1-diarylalkanes from cross-coupling reactions between unactivated aryl boronic esters and benzylic chlorides. Enhanced yields were obtained when 1,3,5-trimethoxybenzene was used as an additive, which is hypothesized to extend the lifetime of the iron-based catalyst. Exceptional enantioselectivities were obtained with challenging ortho-substituted benzylic chlorides.


 Please wait while we load your content...
Please wait while we load your content...