γ-Carboline synthesis enabled by Rh(iii)-catalysed regioselective C–H annulation†
Abstract
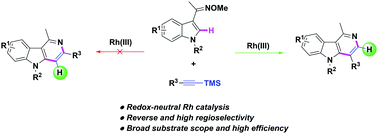
A redox-neutral Rh(III)-catalyzed C–H annulation of indolyl oximes was developed. Relying on the use of various alkynyl silanes as the terminal alkyne surrogates, the reaction exhibited a reverse regioselectivity, thus giving an exclusive and easy way for the synthesis of a wide range of substituent free γ-carbolines at C3 position with high efficiency. Deuterium-labelling experiments and kinetic analysis have preliminarily shed light on the working mode of this catalytic system.


 Please wait while we load your content...
Please wait while we load your content...