Automated solid-phase concatenation of Aib residues to form long, water-soluble, helical peptides†
Abstract
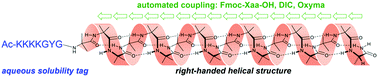
Iterative coupling of 2-aminoisobutyric acid (Aib) has been achieved rapidly and efficiently using automated solid-phase peptide synthesis, employing diisopropylcarbodiimide (DIC) in the presence of ethyl cyanohydroxyiminoacetate (Oxyma). This method has allowed the first total synthesis of the fungal antibiotic Cephaibol D, and enabled the synthesis of water-soluble oligomers of Aib containing up to an unprecedented sequence of 17 consecutive Aib residues. Conformational analysis of the Aib oligomers in aqueous solution shows a length dependence in their CD spectra, with oligomers of more than 14 Aib residues apparently adopting structured helical conformations.


 Please wait while we load your content...
Please wait while we load your content...