Fluoroalkyl sulfides as photoredox-active coupling reagents for alkene difunctionalization†
Abstract
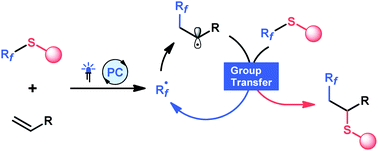
A visible-light-promoted fluoroalkylation-thiolation of alkenes is described. A 4-tetrafluoropyridinylthio fragment serves as a photoredox-active group in the initiation step and undergoes a radical group transfer, which is important for the reaction efficiency. In the primary products, the pyridinylthio substituent may be further functionalized via radical processes or an aromatic substitution reaction.


 Please wait while we load your content...
Please wait while we load your content...