Defluorosilylation of trifluoromethane: upgrading an environmentally damaging fluorocarbon†
Abstract
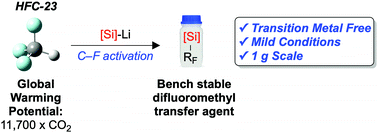
The rapid, room-temperature defluorosilylation of trifluoromethane, a highly potent greenhouse gas, has been achieved using a simple silyl lithium reagent. An extensive computational mechanistic analysis provides a viable reaction pathway and demonstrates the unexpected electrophilic nature of LiCF3. The reaction generates a bench stable fluorinated building block that shows promise as an easy-to-use difluoromethylating agent. The difluoromethyl group is an increasingly important bioisostere in active pharmaceutical ingredients, and therefore our methodology creates value from waste. The potential scalability of the process has been demonstrated by achieving the reaction on a gram-scale.


 Please wait while we load your content...
Please wait while we load your content...