Pd-catalyzed amidation of 1,3-diketones with CO and azides via a nitrene intermediate†
Abstract
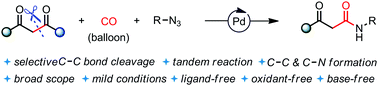
An efficient Pd-catalyzed amidation of 1,3-diketones has been developed using carbon monoxide and organic azides. This reaction provides a step-economic approach to produce β-ketoamides from readily available compounds under mild ligand-, oxidant-, and base-free conditions. The mechanistic studies showed that the reaction occurred through an in situ generated isocyanate intermediate.


 Please wait while we load your content...
Please wait while we load your content...