Synthesis and hetero-Diels–Alder reactions of enantiomerically pure dihydro-1H-azepines†
Abstract
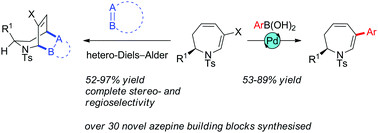
Thermolysis of enantiomerically pure 3-substituted 7,7-dihalo-2-azabicyclo[4.1.0]heptanes in the presence of K2CO3 gives in good yields 2-alkyl-6-halo-1-tosyl-2,3-dihydro-1H-azepines. These undergo highly stereoselective [4+2] cycloaddition reactions with heterodienophiles and arylation/alkenylation under Suzuki conditions.


 Please wait while we load your content...
Please wait while we load your content...