Biosynthesis of oxygenated brasilane terpene glycosides involves a promiscuous N-acetylglucosamine transferase†
Abstract
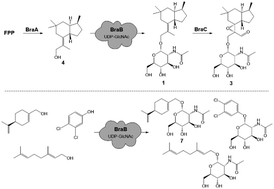
Investigation of the metabolome of the ascomycete Annulohypoxylon truncatum led to the identification of novel oxygenated brasilane glycosides and the revision of the stereochemistry of the brasilane A octahydro-1H-indene core scaffold to trans. The bra biosynthetic gene cluster containing five genes (braA–braE) was identified and verified by heterologous expression experiments in Aspergillus oryzae demonstrating that BraC is a multifunctional P450 monooxygenase. In vitro studies of BraB revealed it to be a very rare fungal UDP-GlcNAc dependent N-acetylglucosamine transferase. UDP-glucose is also accepted as a donor, and a broad acceptor substrate tolerance for various primary and secondary alcohols was observed.


 Please wait while we load your content...
Please wait while we load your content...