Fragment-oriented synthesis: β-elaboration of cyclic amine fragments using enecarbamates as platform intermediates†
Abstract
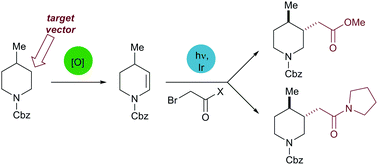
A strategy for the β-sp3 functionalisation of cyclic amines is described. Regioselective conversion of protected amines to enecarbamates is achieved through electrochemical oxidation; these intermediates can be derivatised by functionalised alkyl halides under photoredox catalysis. The potential of the methods is highlighted by direct growth of a DCP2B-binding fragment.


 Please wait while we load your content...
Please wait while we load your content...