Astatine partitioning between nitric acid and conventional solvents: indication of covalency in ketone complexation of AtO+†
Abstract
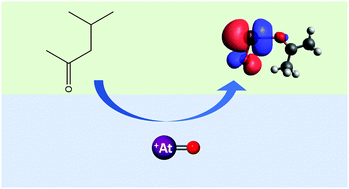
Astatine-211 has been produced at Texas A&M University on the K150 cyclotron, with a yield of 890 ± 80 MBq through the 209Bi(α,2n)211At reaction via an 8 h bombardment with a beam current of 4–8 μA and an α-particle beam energy of 28.8 MeV. The target was then dissolved in HNO3 and the extraction of 211At was investigated into a variety of organic solvents in 1–3 M HNO3. Extraction of 211At with distribution ratios as high as 11.3 ± 0.6, 12.3 ± 0.8, 42.2 ± 2.2, 69 ± 4, and 95 ± 6 were observed for diisopropyl ether, 1-decanol, 1-octanol, 3-octanone, and methyl isobutyl ketone, respectively, while the distribution ratios for 207Bi were ≤0.05 in all cases. The extraction of 211At into both methyl isobutyl ketone and 3-octanone showed a strong, linear dependence on the HNO3 initial aqueous concentration and better extraction than other solvents. DFT calculations show stronger binding between the carbonyl oxygen of the ketone and the At metal center.


 Please wait while we load your content...
Please wait while we load your content...
