Does deamidation affect inhibitory mechanisms towards amyloid protein aggregation?†
Abstract
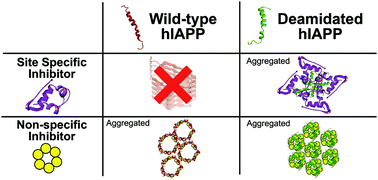
Deamidated amyloid proteins have been shown to accelerate fibril formation. Herein, the results show the inhibition performance and the interaction site between site-specific inhibitor and amyloid protein are significantly influenced by deamidation; while the inhibition mechanism of non-site specific inhibitor shows no significant disruption caused by amyloid protein deamidation.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...