Multicomponent benzannulation of allylic P-ylides with isocyanates or aldehydes for construction of anilines and biaryls†
Abstract
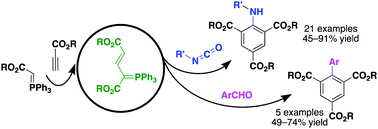
The reactivity of allylic phosphorus ylides generated in situ from alkoxycarbonylmethylenephosphoranes and propiolates is investigated toward isocyanates and aromatic aldehydes, which leads to one-pot multicomponent benzannulations for efficient synthesis of polysubstituted anilines and biaryls, respectively. The mechanism may involve a tandem [2+2] cycloaddition/fragmentation/Wittig/cyclization/elimination/aromatization sequence.


 Please wait while we load your content...
Please wait while we load your content...