The copper(ii)-catalyzed and oxidant-promoted regioselective C-2 difluoromethylation of indoles and pyrroles†
Abstract
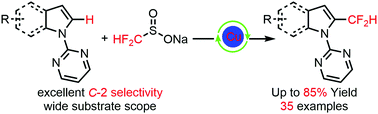
A novel and efficient approach for the highly selective C-2 difluoromethylation of indole derivatives was developed by using sodium difluoromethylsulfinate (HCF2SO2Na) as the source of difluoromethyl groups and a Cu(II) complex as the catalyst. Various substrates were well tolerated in this transformation and the desired products were obtained in moderate to good yields. Moreover, the late-stage C-2 difluoromethylation of bioactive molecules containing an indole ring was achieved in good yields. Generally, this reaction features excellent functional group compatibility, broad substrate scope and excellent C-2 selectivity.


 Please wait while we load your content...
Please wait while we load your content...