Exploration of chiral diastereomeric spiroketal (SPIROL)-based phosphinite ligands in asymmetric hydrogenation of heterocycles†
Abstract
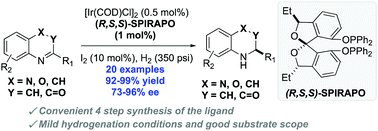
New and readily available chiral SPIROL-based diphosphinite ligands (SPIRAPO) have been prepared and employed for iridium-catalyzed asymmetric hydrogenations of quinolines, quinoxalines and 2H-1,4-bezoxazin-2-ones. While the structurally similar (R,R,R)-SPIRAPO and (R)-SPINOL-based phosphinites were not the best ligands for these transformations, the (S,R,R)-diastereomer of SPIRAPO was found to be highly effective ligand for the reduction of 20 different heterocyclic systems with loadings as low as S/C = 10 000. This dearomatizative hydrogenation provided direct access to optically active tetrahydroquinolines in high enantioselectivities (up to 94% ee) and excellent yields (up to 99%), and was used to generate 1.75 g of natural alkaloid (−)-(R)-angustureine. This protocol was subsequently extended to achieve asymmetric hydrogenation of quinoxalines and 2H-1,4-benzoxazin-2-ones in good to excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...