Merging alkenyl C–H activation with the ring-opening of 1,2-oxazetidines: ruthenium-catalyzed aminomethylation of enamides†
Abstract
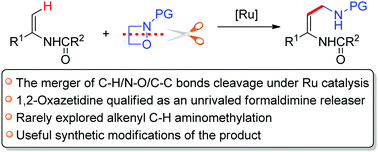
1,2-Oxazetidines have been utilized as formaldimine precursors for the direct aminomethylation of enamides under a Ru(II) species. By merging alkenyl C–H activation with ring-opening of 1,2-oxazetidines, this efficient protocol provides a facile and novel approach to synthesize Z-selective aminomethyl substituted enamides. Furthermore, two exemplified synthetic elaborations highlight the potential of this transformation.


 Please wait while we load your content...
Please wait while we load your content...