Fluorogenic iminosydnones: bioorthogonal tools for double turn-on click-and-release reactions†
Abstract
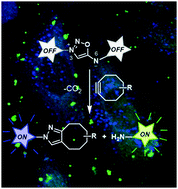
In this article, we report the synthesis and use of iminosydnone-based profluorophores as bioorthogonal cleavable linkers for imaging applications. These linkers react with cycloalkynes via subsequent [3+2] cycloaddition and retro Diels–Alder reactions, allowing simultaneous release of two dyes in biological media.


 Please wait while we load your content...
Please wait while we load your content...