Biliazine: a ring open phthalocyanine analog with a meso hydrogen bond†
Abstract
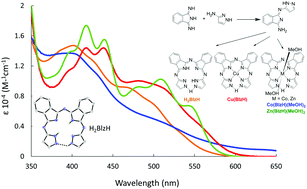
The hemiporphyrazine type chelate and phthalocyaninine analog biliazine (H2BlzH) and its metal complexes can be synthesized in one-step from a monosubstituted diiminisoindoline (DII). This singly substituted precursor is produced from the reaction of unsubstituted DII and 2-aminopyrazole. The biliazine macrocycle is a tetradentate chelate where the ring is closed via a strong hydrogen bond at the meso position. Although the hydrogen bond closes the ring, there is little evidence for 18-electron type annulene aromaticity across the macrocycle, as seen in the UV-visible and MCD spectra and supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...