Synergism of anisotropic and computational NMR methods reveals the likely configuration of phormidolide A†
Abstract
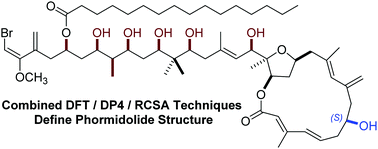
Characterization of the complex molecular scaffold of the marine polyketide natural product phormidolide A represents a challenge that has persisted for nearly two decades. In light of discordant results arising from recent synthetic and biosynthetic reports, a rigorous study of the configuration of phormidolide A was necessary. This report outlines a synergistic effort employing computational and anisotropic NMR investigation, that provided orthogonal confirmation of the reassigned side chain, as well as supporting a further correction of the C7 stereocenter.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...