Copper-catalyzed synthesis of aminoquinolines from β-(2-aminophenyl)-α,β-ynones using DMF as dual synthon†
Abstract
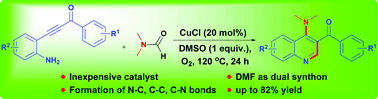
A new and efficient method has been developed for the synthesis of 4-aminoquinoline through aerobic Cu(I)-catalyzed cyclization of β-(2-aminophenyl)-α,β-ynones. Under the optimized conditions, DMF could serve as a methine source to introduce C2 carbon and a nitrogen source to incorporate amino functionality in the 4th position. Mechanistic studies using 13C- and DMF-d7 revealed that the methine group was derived from a methyl substituent.


 Please wait while we load your content...
Please wait while we load your content...