A short peptide that preferentially binds c-MYC G-quadruplex DNA†
Abstract
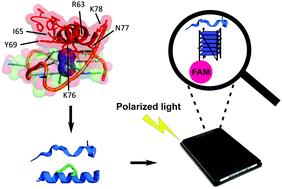
G-quadruplexes (G4s) are non-canonical DNA secondary structures. The identification of selective tools to probe individual G4s over the ∼700 000 found in the human genome is key to unravel the biological significance of specific G4s. We took inspiration from a crystal structure of the bovine DHX36 helicase bound to the G4 formed in the promoter region of the oncogene c-MYC to identify a short peptide that preferentially binds MYC G4 with nM affinity over a small panel of parallel and non-parallel G4s tested.


 Please wait while we load your content...
Please wait while we load your content...