Direct access to pentenedinitriles via Ni-catalyzed dihydrocyanation of 1,3-enynes†
Abstract
A highly regio- and stereoselective dihydrocyanation of 1,3-enynes was implemented by nickel/diphosphine catalysts. This reaction represents the first example of Ni-catalyzed dihydrocyanation of 1,3-enynes using TMSCN and MeOH as HCN surrogates. In this transformation, the main key feature is a multistep combination by using a single catalyst system. We observed a critical influence of the HCN source on the dihydrocyanation reaction. Moreover, the double hydrocyanation products were conveniently converted to poly-substituted pyridines.
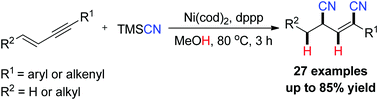


 Please wait while we load your content...
Please wait while we load your content...