Zinc(ii)-catalyzed intramolecular hydroarylation-redox cross-dehydrogenative coupling of N-propargylanilines with diverse carbon pronucleophiles: facile access to functionalized tetrahydroquinolines†
Abstract
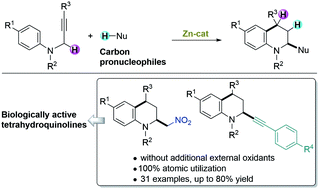
Zinc(II)-catalyzed intramolecular hydroarylation-redox cross-dehydrogenative coupling of N-propargylanilines with two types of carbon pronucleophiles (nitromethane as a sp3 carbon pronucleophile and phenylacetylenes as sp carbon pronucleophiles) proceeded to give the 2-substituted tetrahydroquinolines in good yields with 100% atomic utilization without any additional external oxidants.


 Please wait while we load your content...
Please wait while we load your content...