Conversion of racemic alcohols to optically pure amine precursors enabled by catalyst dynamic kinetic resolution: experiment and computation†
Abstract
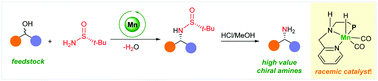
An unprecedented base metal catalysed asymmetric synthesis of α-chiral amine precursors from racemic alcohols is reported. This redox-neutral reaction utilises a bench-stable manganese complex and Ellman's sulfinamide as a versatile ammonia surrogate. DFT calculations explain the unusual finding of the highly stereoselective transformation enabled by a catalyst that undergoes an unusual dynamic kinetic resolution.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...