Trivalent metal complex geometry of the substrate governs cathepsin B enzymatic cleavage rate†
Abstract
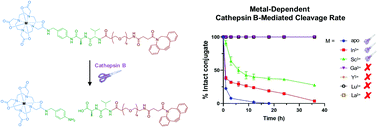
The lysosomal protease cathepsin B recognizes defined, short peptide sequences, providing means for effective, targeted drug release. Here, we show that the introduction of a coordination complex adjacent to the cleavage sequence allows us to tune enzymatic cleavage rate by varying the complexed, trivalent metal ion.


 Please wait while we load your content...
Please wait while we load your content...