Direct enantioselective allylic substitution of 4-hydroxycoumarin derivatives with branched allylic alcohols via iridium catalysis†
Abstract
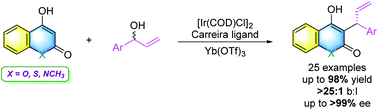
A highly efficient direct asymmetric allylic substitution (AAS) reaction of 4-hydroxycoumarin derivatives with branched allylic alcohols was realized by combining a chiral iridium complex catalyst with a Lewis acid under mild reaction conditions, delivering various chiral allylation products in remarkably high yields and excellent enantioselectivities. The salient features of this transformation include mild reaction conditions, general substrate scope, good functional group tolerance, high yields, excellent selectivities and easy scale-up. Furthermore, the obtained products can be readily transformed into several kinds of bioactive compounds.


 Please wait while we load your content...
Please wait while we load your content...