Synthesis of amino-diamondoid pharmacophores via photocatalytic C–H aminoalkylation†
Abstract
We report a direct C–H aminoalkylation reaction using two light-activated H-atom transfer catalyst systems that enable the introduction of protected amines to native adamantane scaffolds with C–C bond formation. The scope of adamantane and imine reaction partners is broad and deprotection provides versatile amine and amino acid building blocks. Using readily available chiral imines, the enantioselective synthesis of the saxagliptin core and rimantadine derivatives is also described.
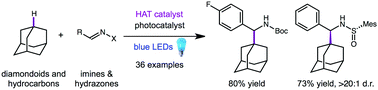


 Please wait while we load your content...
Please wait while we load your content...