BF3-Etherate-catalyzed tandem reaction of 2-formylarylketones with electron-rich arenes/heteroarenes: an assembly of isobenzofurans†
Abstract
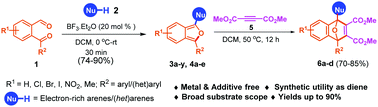
An efficient and BF3·Et2O-catalyzed chemoselective synthesis of diversified 1,3-diarylisobenzofuran in a high yield has been described. The reaction proceeds through sequential hydroarylation–cyclization between 2-formylarylketones and electron-rich arenes/heteroarenes. Advantageous features of the developed methodology include operational simplicity, a broad substrate scope, and applicability towards gram scale synthesis. The utility of isobenzofuran derivatives as the diene was extended to the synthesis of [4+2] cyclo-adducts with DMAD and the synthesis of 1,2-dicarbonylarenes in good yields.


 Please wait while we load your content...
Please wait while we load your content...