Site-selective synthesis of 1,3-dioxin-3-ones via a gold(i) catalyzed cascade reaction†
Abstract
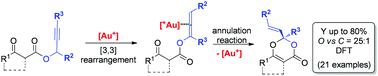
A novel gold(I)-catalyzed protocol for the synthesis of 4H-1,3-dioxin-3-ones is presented. The protocol exploits a metal induced cascade sequence involving a [3,3]-sigmatropic rearrangement followed by regioselective O-annulation reactions. A wide range of oxygen-based heterocyclic scaffolds (21 examples) were achieved in excellent yield (up to 80%) and a detailed computational investigation as well as deuterium-labelling investigations enabled all the plausible reaction pathways to be mapped and the rationalization of the recorded regioselectivity.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...