Controllable synthesis of 2- and 3-aryl-benzomorpholines from 2-aminophenols and 4-vinylphenols†
Abstract
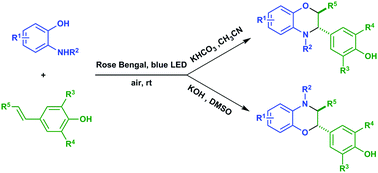
We present herein a method for the controllable synthesis of 3-aryl-benzomorpholine and 2-aryl-benzomorpholine cycloadducts via cross-coupling/annulation between electron-rich 2-aminophenols and 4-vinylphenols. Molecular oxygen was successfully used in the reaction as the terminal oxidant and the complete inversion of chemoselectivity was achieved by the adjustment of the solvents and bases at room temperature.


 Please wait while we load your content...
Please wait while we load your content...