B(C6F5)3- and HB(C6F5)2-mediated transformations of isothiocyanates†
Abstract
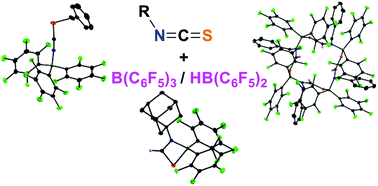
This contribution reports on the reactivity of isothiocyanates towards the boranes B(C6F5)3 and HB(C6F5)2. The reactions of alkyl-substituted isothiocyanates with B(C6F5)3 were found to result in rearrangement reactions to yield stable thiocyanate–B(C6F5)3 adducts. Treatment of isothiocyanates with HB(C6F5)2 leads to 1,2-hydroboration and thus, B,N,C,S heterocycles are formed, which react further under non-inert conditions. Hydrolysis of the hydroboration products leads to a new access to thioformamides.


 Please wait while we load your content...
Please wait while we load your content...