Catalytic and enantioselective oxa-Piancatelli reaction using a chiral vanadium complex†
Abstract
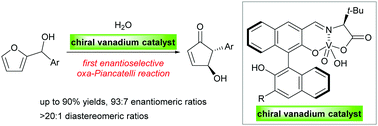
An enantioselective oxa-Piancatelli reaction was established for the first time using a chiral vanadium(V) catalyst. The dual Brønsted and Lewis acid properties of the vanadium catalyst afforded 4-hydroxycyclopent-2-enone derivatives in up to 90% yields and with 93 : 7 enantiomeric ratios, as well as >20 : 1 diastereomeric ratios.


 Please wait while we load your content...
Please wait while we load your content...