Reductive umpolung for asymmetric synthesis of chiral α-allenic alcohols†
Abstract
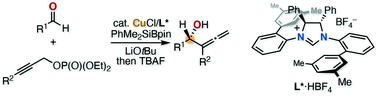
In this communication, we report a chiral copper/NHC-catalyzed reaction between aromatic aldehydes and propargylic phosphates using a silylboronate, providing enantioenriched chiral α-allenic alcohols with complete regioselectivity and moderate to high enantioselectivity. The reaction pathway involves the catalytic formation of a nucleophilic α-alkoxylalkylcopper(I) species from aldehydes followed by its SN2′ type substitution reaction with propargylic phosphates.


 Please wait while we load your content...
Please wait while we load your content...