Intramolecularly stapled amphipathic peptides via a boron–sugar interaction†
Abstract
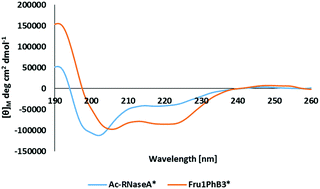
Amadori products (deoxyfructosyllysine derivatives) that can selectively interact with phenylboronic acids and borate ions were synthesized. The intramolecular interactions between the fructosyl moiety and phenylboronic acid incorporated into various positions of the peptide chain were investigated using high-resolution mass spectrometry (HR–MS), circular dichroism (CD), and nuclear magnetic resonance (NMR).


 Please wait while we load your content...
Please wait while we load your content...