Disulfides as mercapto-precursors in nucleophilic ring opening reaction of polymeric epoxides: establishing equimolar stoichiometric conditions in a thiol–epoxy ‘click’ reaction†
Abstract
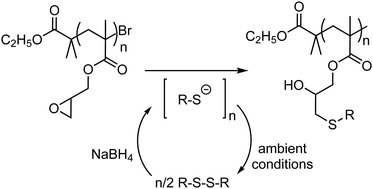
The base-catalyzed oxirane ring opening reaction with thiol nucleophiles is frequently employed for post-polymerization modification of polymeric glycidyl scaffolds. Due to various beneficial attributes, it is often referred to as a ‘click’ reaction. However, the tendency of the free thiol molecules to undergo oxidative dimerization through the formation of a disulfide bond under ambient conditions results in partial consumption of the sulfhydryl precursors. Therefore, an excess of the thiol precursors is typically used to counterbalance the side-reaction. This violates the equimolar stoichiometry conditions required for ‘click’ reactions in the context of polymer synthesis. Here, we show that commercially available disulfides can be used to generate the necessary thiolate nucleophiles in situ through the reduction of the SS-bond with sodium borohydride. Such activation strategy eliminates the sulfhydryl oxidation mechanism to disulfides and ensures that the post-synthesis functionalization of epoxy polymers can be performed under equimolar ‘click’ conditions.


 Please wait while we load your content...
Please wait while we load your content...