Construction of multiple bonds via a domino reaction of trifluoroacetimidoyl nitriles with in situ generated bis-nucleophiles†
Abstract
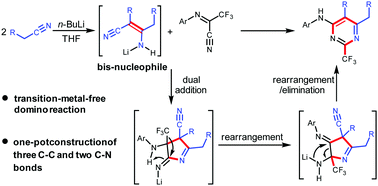
A transition-metal-free double addition/double rearrangement domino reaction affording CF3-substituted pyrimidines was developed, which enables the one-pot construction of five new bonds, namely three C–C bonds and two C–N bonds. The keys to achieve this highly efficient reaction include the delicate design of the bis-nucleophiles in situ generated from the dimerization of alkyl nitriles and the use of trifluoroacetimidoyl nitriles containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, and CF3 groups as the reactant. The mechanistic studies by the experiments and DFT calculations reveal that the transformation involves two addition and two unprecedented rearrangement processes.
N, and CF3 groups as the reactant. The mechanistic studies by the experiments and DFT calculations reveal that the transformation involves two addition and two unprecedented rearrangement processes.


 Please wait while we load your content...
Please wait while we load your content...