Chiral Brønsted acid-catalyzed dynamic kinetic resolution of atropisomeric ortho-formyl naphthamides†
Abstract
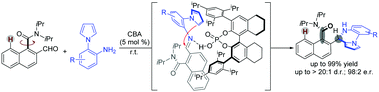
Despite the widespread use of naphthamide atropisomers in biologically active compounds and asymmetric catalysis, few catalytic methods have succeeded in the enantioselective synthesis of these compounds. Herein, a chiral Brønsted acid (CBA) catalysis strategy was developed for readily scalable dynamic kinetic resolution of challenging ortho-formyl naphthamides with pyrrolylanilines. The chiral axis of the atropisomeric amide and a stereogenic center were simultaneously established for a new family of potential biologically active pyrrolopyrazine compounds with high enantio- and diastereoselectivities (up to >20 : 1 d.r. and 98 : 2 e.r.). Epimerization experiments of its derivatives reveal that the N-substitution of the nearby stereogenic center could affect the configurational stability of the axially chiral aromatic amides. These results might be useful for the construction of other kinds of novel axially chiral molecules with a low rotational barrier.


 Please wait while we load your content...
Please wait while we load your content...