Atroposelective synthesis of configurationally stable nonbiaryl N–C atropisomers through direct asymmetric aminations of 1,3-benzenediamines†
Abstract
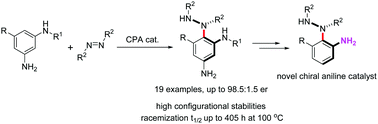
A highly atroposelective synthesis of nonbiaryl N–C atropisomers was achieved via direct aminations of 1,3-benzenediamines with azodicarboxylates enabled by chiral phosphoric acid catalysis. A series of N-substituents, benzene-substituents and azodicarboxylates were well tolerated, generating N–C atropisomers with high configurational stability. The facile derivatizations and utilizations of the chiral products as novel chiral organocatalysts demonstrate the value of these reactions.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...