Dehydroxyalkylative halogenation of C(aryl)–C bonds of aryl alcohols†
Abstract
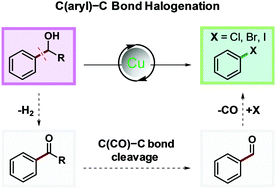
We herein report Cu mediated side-directed dehydroxyalkylative halogenation of aryl alcohols. C(aryl)–C bonds of aryl alcohols were effectively cleaved, affording the corresponding aryl chlorides, bromides and iodides in excellent yields. Aryl alcohols could serve as both aromatic electrophilic and radical synthetic equivalents during the reaction.


 Please wait while we load your content...
Please wait while we load your content...