Sulfoxide synthesis from sulfinate esters under Pummerer-like conditions†
Abstract
A facile synthetic method for the preparation of allyl sulfoxides by S-allylation of sulfinate esters proceeds through sulfonium intermediates without [3,3]-sigmatropic rearrangement and further Pummerer-type reactions of the resulting allyl sulfoxides. On the basis of the plausible reaction mechanism involving sulfonium salt intermediates, S-alkynylation and S-arylation were also accomplished.
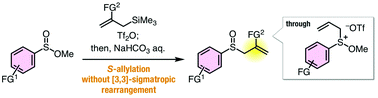


 Please wait while we load your content...
Please wait while we load your content...